Introduction
Inflammation is a protective response involving host cells, blood vessels, and proteins and other mediators, that is intended to eliminate the initial cause of cell injury, as well as the necrotic cells and tissues resulting from the original insult, and to initiate the process of repair. Inflammation may occur due to many causes, including infection, trauma, physical and chemical agents, necrosis, foreign bodies, and immune reactions. It is a complex mechanism that involves many chemical mediators with multiple functions. Also, the same mediator may have different effects on different tissues. Although, inflammation is a protective response; however, it may significantly damage the host tissues also. For example, in periodontal diseases, present evidence strongly suggests that the host response is equally responsible for periodontal destruction as the microbial infection. There are various components of the inflammatory process. These include leukocytes and plasma proteins that normally circulate in the blood and are brought to the site of inflammation, the resident cells of vascular walls and the cells and proteins of the extracellular matrix.
Signs of inflammation
The external manifestations of inflammation are often called its cardinal signs of inflammation. The four principal effects of inflammation (rubor, tumor, calor et dolor) were described nearly 2,000 years ago by the Roman Aulus Cornelius Celsus, more commonly known as Celsus. The description of these signs is as follows,
Redness (rubor): An acutely inflamed tissue appears red, due to dilatation of small blood vessels within the damaged area (hyperemia).
Swelling (tumor): Swelling results from edema, the accumulation of fluid in the extravascular space as part of the inflammatory fluid exudate, and to a much lesser extent, from the physical mass of the inflammatory cells migrating into the area.
Heat (calor): Increase in temperature is readily detected in the skin. It is due to increased blood flow (hyperemia) through the region, resulting in vascular dilation and the delivery of warm blood to the area.
Pain (dolor): Pain results partly from the stretching and distortion of tissues due to inflammatory edema and, in part from some of the chemical mediators of acute inflammation, especially bradykinin and some of the prostaglandins.
Loss of function (functio laesa): Loss of function, a well-known consequence of inflammation, was added by Virchow (1821-1902) to the list of features described in Celsus’ written work. Movement of an inflamed area is inhibited by pain, either consciously or by reflexes, while severe swelling may physically immobilize the affected area.

Types of inflammation
Inflammation is the first line of defense against injury or infection. It can be classified as acute or chronic. Acute inflammation is of relatively short duration (hours to days) and is primarily characterized by exudation of fluid and plasma proteins, as well as a neutrophilic infiltration. Chronic inflammation is of longer duration (days to years) and is characterized by mononuclear infiltration, vascular prolifera-tion, and scarring.
Acute inflammation:
The acute inflammation involves an immediate and early response to tissue injury (physical, chemical, microbiologic, etc.). It has two major components,
Vascular changes:
There are specific vascular changes that occur during acute inflammation. The vascular events of the acute inflammatory response involve three main processes:
1. Changes in vessel caliber and, consequently, blood flow (hemodynamics),
2. Increased vascular permeability, and
3. Formation of the fluid exudate.
After tissue injury, the earliest event of an inflammatory response is temporary vasoconstriction, i.e. narrowing of blood vessels caused by contraction of smooth muscle in the vessel walls, which can be seen as blanching of the skin. It is followed by an acute vascular response, within seconds of the tissue injury and last for some minutes. This results from vasodilation and increased capillary permeability due to alterations in the vascular endothelium, which leads to increased blood flow (hyperemia) that causes redness (erythema) and the entry of fluid into the tissues (edema). This response of the body to injury is also called as the “wheal and flare reaction”.
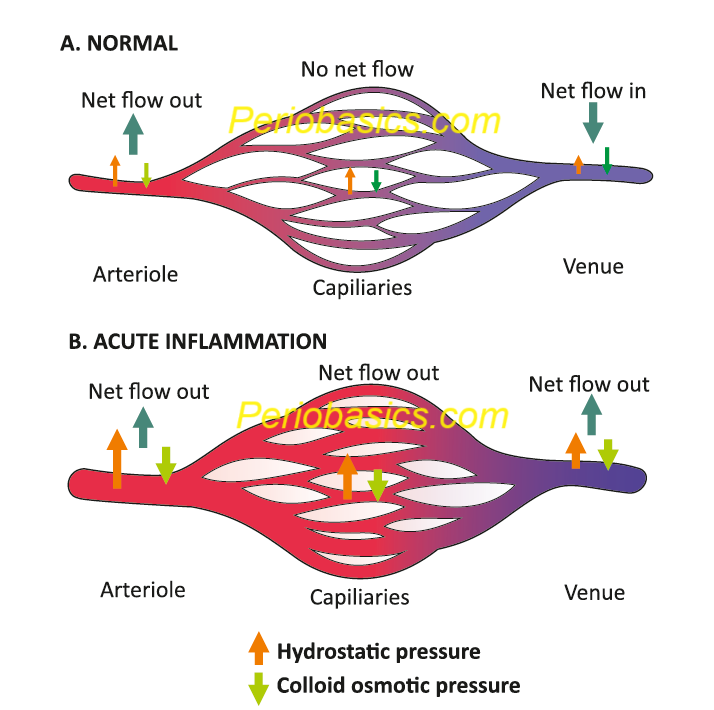
The microcirculation consists of the network of small capillaries lying between arterioles, which have a thick muscular wall, and thin-walled venules. There are no smooth muscles in the capillaries to control their caliber and they are so narrow that red blood cells must pass through them in single file. The flow to capillary bed is controlled by smooth muscle of arteriolar walls. During inflammation, the blood flow to the injured area may increase up to ten-fold, as vessels dilate (Figure 37.1). These changes are mediated by ………………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Cellular events:
These include emigration of the leukocytes from the circula-tion and accumulation in the focus of injury. The principal leukocytes in acute inflammation are neutrophils. This is caused by the slowing of blood, which causes red cells to clump and flow in the central regions of blood flow while white cells move to the edges where they adhere to endothelial cells. The white blood cells first attach themselves to the endothelial cells within the blood vessels (margination) and then cross into the surrounding tissue (diapedesis or trans-endothelial migration).
Mechanism of transendothelial migration
As already stated, under normal condition, the blood cells are confined to the central stream in the blood vessel and not in the peripheral or plasmatic zone. However, following an injury, the acute inflammatory reaction is initiated which results in the loss of intravascular fluid and increase in plasma viscosity with slowing of blood flow at the site of acute inflammation. The reduced flow rate of the blood allows neutrophils to flow in the plasmatic zone. Once, the neutrophils come near the wall of the blood vessel, the process of transendothelial migration is initiated. There are a number of receptor molecules that are involved in the process of leukocyte migration from inside the blood vessel to outside in the connective tissue. The knowledge of these receptors is essential to understand the mechanism of transendothelial migration.
Molecules involved in trans-endothelial migration:
The role of the majority of the following molecules in the trans-endothelial migration of leukocytes has been demonstrated by blocking the respective receptors by antibodies.
Selectins:
Selectins belong to a family of transmembrane molecules, expressed on the surface of leukocytes and activated endothelial cells. They are of 3 types; L-selectin, P-selectin, and E-selectin. The smallest of these is L-selectin, which is found on most leukocytes. P-selectin, the largest selectin, is expressed on activated platelets and endothelial cells primarily. E-selectin is expressed on activated endothelium during chemically or cytokine-induced inflammation. These contain an N-terminal extracellular domain with structural homology to calcium-dependent lectins, followed by a ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Integrins:
These are transmembrane adhesive heterodimeric glycopro-teins, made up of α and β subunits that function as receptors for the extracellular matrix. β subunit is known as CD18 and the α subunit is known as CD11. The α subunit is found in 4 forms, namely a, b, c, and d. So, based on α subunit variability, these glycoproteins are classified as,
CD11a/CD18, also known as lymphocyte function-associated antigen-1 (LFA-1),
CD11b/CD18, also known as macrophage receptor-1 (MAC-1),
CD11c/CD18 (p150, 95/CR4), and
CD11d/CD18 (αDβ2).
The principal receptors for ICAM-1 are the β integrins LFA-1 and MAC-1, and those for VCAM-1 are the integrins α4β1 and α4β7.
Intercellular adhesion molecules (ICAM-1 & ICAM-2):
These molecules perform many important tasks during trans-endothelial migration. They belong to the immunoglobulin superfamily and represent endothelial ligands for the leukocyte β-2 integrin, LFA-1. ICAM-1 is constitutively expressed on endothelial cells, platelets, and most leukocytes whereas, ICAM-2 appears to be concentrated at endothelial cell junctions.
Platelet endothelial cell adhesion molecule-1 (PEC AM-1) or CD-31:
These molecules help in the endothelial cell to cell adhesion via homophilic interactions and they help in transendothelial migration through endothelium-leukocyte interactions. PECAM-1 homophilic interactions allow leukocytes to emigrate through the endothelial barrier. These belong to the immunoglobulin gene superfamily and are expressed on leukocytes, platelets, neutrophils, monocytes, and selected T-cell subsets.
Junctional adhesion molecules (JAMs):
JAMs are the members of the immunoglobulin gene superfamily. JAM proteins are localized in the intercellular junctions of polarized endothelial and epithelial cells. They are found in three forms JAM-A, JAM-B, and JAM-C. JAM-A is expressed at epithelial tight junctions and intercellular borders of endothelial cells, as well as on the surfaces of megakaryocytes. JAM-B and JAM-C are believed to be invo-lved in leukocyte adhesion, transmigration, and interactions between different cell subsets during inflammation. Although JAM-A normally engages in homophilic adhesion, during inflammation it can bind to CD11a/CD18 on the leukocyte.
Vascular cell adhesion molecule-1 (VCAM-1):
It is an adhesion molecule that is not constitutively expressed on endothelial cells, but is upregulated by chemokines. This molecule has been shown to interact with monocytes and lymphocytes and participates in leukocyte transmigration during the inflammatory response.
VE-cadherin:
VE-cadherin is a transmembrane protein that establishes homotypic calcium-dependent interactions with its extra-cellular domain. It is one of the major components of adherence junctions. Although, many proteins have been implicated in endothelial cell-cell adhesion; VE-cadherin has a central role in the regulation of the integrity of the ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
CD99:
CD99 is a 32 kD, a highly O-glycosylated molecule that is expressed on the surfaces of most leukocytes and is concentrated at the borders between confluent endothelial cells. Similar to PECAM-1, CD99 functions in a homophilic manner in the transmigration of leukocytes but CD99 regulates a later step in this process as compared to PECAM.
CD99L2 (CD99-like molecule 2):
It represents a protein of unknown function with moderate sequence homology to CD99, which is expressed on leukocytes and endothelial cells. Similar to PECAM, the homophilic interaction between CD99 at the endothelial cell border and CD99 on monocytes and neutrophils is required for transmigration.
Steps in transendothelial migration of leukocytes:
Slow rolling,
Adhesion strengthening,
Intra-luminal crawling,
Transendothelial migration,
Migration through the basement membrane, and
Interstitial migration
Step by step mechanism of transendothelial migration:
1. As already stated, inflammation is a protective response of the body to any insult, which is manifested by the release of a variety of pro-inflammatory mediators from resident leukocytes and mast cells, including cytokines like IL-1β, TNF-α etc. Complement components like C3a and C5a are also important initiators of transendothelial migration of leukocytes.
2. These mediators stimulate endothelial cells to express P-selectin and E-selectin on their luminal surfaces. Initial tethering and rolling are mediated by P-, E- and L-selectins (Figure 37.2). The initial rolling brings the leukocyte into proximity with endothelial cells, where it can be activated by luminal surface-bound chemokines or lipid chemo-attractants (for example, platelet activating factor- PAF). Recent works suggest that not only selectins, but also integrins such as LFA-1 and MAC-1, support leukocyte rolling.
3. After the initial tethering and rolling, the intercellular adh-esion molecule-1 (ICAM-1) and vascular cell adhesion molecule (VCAM-1) are expressed. Their binding to activated integrins probably contributes to adhesion stabilization and cell motility. In addition to mediating adhesion, integrins also generate intracellular signals that regulate various cellular functions.
4. Subsequently, leukocytes crawl inside the blood vessels in a MAC-1 and ICAM-1 dependent manner, seeking the preferred sites for diapedesis. After crawling, leukocytes migrate to a nearby endothelial border and squeeze between the tightly opposed endothelial cells to the underlying basement membrane in the next process of transendothelial migration (also called as diapedesis). PECAM-1 (CD31) and CD99 act at sequential steps as the leukocyte crosses the endothelial barrier. Two main routes of leukocyte transendothelial migration are ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Most of the recently published studies identify, however, the para-cellular migration route as the main mechanism by which leukocytes emigrate from the intravascular compartment into the interstitium. Many cell contact proteins such as PECAM-1, members of the JAM family (JAM-A, JAM-B, and JAM-C), CD99, and ICAM-2 etc. are involved in the para-cellular migration of leukocyte into the extracellular matrix.
5. Ultimately, the leukocyte enters the extracellular matrix and under the chemoattractant gradient reaches the site of inflammation, where it performs its various functions. The chemoattractants may be exogenous or endogenous in origin. These include,
Bacterial products, particularly peptides with N-formyl-methionine,
Cytokines, especially those of the chemokine family,
Components of the complement system, particularly C5, and
Products of the lipoxygenase pathway of arachidonic acid metabolism, particularly leukotriene B4 (LTB4).
Neutrophil-derived products and their functions
The activated neutrophils synthesize and secrete cytokines, chemokines, leukotrienes and prostaglandins, and by virtue of their accumulation in large numbers within the inflammatory tissue, they may contribute significantly to the local production of inflammatory mediators. The activated neutro-phils have been shown to produce IL-1, IL-1RA, IL-6, IL-12, TGF-β, and TNF-α. Furthermore, neutrophils also synthesize and secrete leukotrienes and prostaglandins, especially leukotriene B4 (LTB4) and prostaglandin E2 (PGE2), which are synthesized from arachidonic acid by lipoxygenases and cyclooxygenases pathways, respectively. Neutrophils also secrete matrix metalloproteinases (MMP-8 and MMP-9) which are involved in the degradation of connective tissue. These mediators along with causing inflammatory changes in the tissue, also activate other immune cells.
Chemical mediators of acute inflammation
- Histamine (derived from mast cells and platelets).
- Serotonin (derived from platelets).
- Plasma protein systems:
- Kinin system.
- Complement system.
- Clotting/Fibrinolytic System.
- Eicosanoids derived from arachidonic acid metabolism:
- Prostaglandins.
- Leukotrienes.
- Interleukin 1 (IL-1).
- Interleukin 6 (IL-6).
- Interleukin 1 (IL-1).
- Tumor necrosis factor (TNF).
Products of phagocytosis
Oxygen-derived free radicals.
Cationic proteins.
Neutral proteases.
Nitric oxide (NO)
Histamine:
Serotonin:
Prostaglandins:
Leukotrienes:
Cytokines:
Chemokines:
Reactive oxygen species:
Nitric oxide (NO):
Components of complement system:
Kinins:
Leukocyte activation and phagocytosis
The neutrophils that have transmigrated to the connective tissue have to be activated to perform their functions. The leukocytes possess various receptors to sense the presence of microbes, dead cells, and foreign substances. Once these receptors are engaged by the leukocytes, they get activated and perform their normal defensive function. They perform the function of phagocytosis, which involves the following steps,
- Receptors on the cell surface recognize and bind to the pathogen;
- Signals are generated that induce actin polymerization under the membrane at the site of contact;
- Actin-rich membrane extensions reach out around the particle;
- The membranes fuse behind the particle, pulling it in toward the center of the cell; and
- Phagosome matures via a series of membrane fusion and fission events to become a phagolysosome. The phagoly-sosome is an acidic, hydrolytic compartment in which the pathogen is killed and digested in preparation for antigen presentation.
The destruction of the phagocytosed particles is done be following mechanisms,
Oxygen radicals:
Phagocyte oxidase is a complex of proteins in the membrane of a phagolysosome which generates oxygen radicals in the phagosome. A single electron is taken from NADPH and added to oxygen, partially reducing it. The resulting highly reactive molecules react with proteins, lipids and other biological molecules.
Nitric oxide:
Nitric oxide is synthesized by nitric oxide synthase, a reactive substance that reacts with superoxide to create further molecules that damage various biological molecules.
Antimicrobial proteins:
Lysosomes contain several proteases, including a broad spectrum enzyme, elastase, which is important or even essential for killing various bacteria. Another antimicrobial protein is lysozyme, which attacks the cell walls of certain (gram-positive) bacteria.
Antimicrobial peptides:
Defensins and certain other peptides attack bacterial cell membranes.
Binding proteins:
Lactoferrin binds iron ions, which are necessary for the growth of bacteria. Another protein binds vitamin B12.
Hydrogen ion transport:
Transporters for hydrogen ions (a second role of the oxidase) acidify the phagolysosome, which kills various microorga-nisms and is important for the action of the proteases described above.
Outcome of acute inflammation
The outcome of acute inflammation primarily depends on the nature and intensity of the injury, the site, and tissue affected, and the ability of the host to mount a response. Broadly, there are three outcomes of acute inflammation,
Complete resolution:
It occurs when the injury is short lived and has caused minimal tissue damage. The complete resolution results in restoration of structural and functional normalcy. The inflammatory response is slowly reduced and normalcy in tissue hemo-dynamics is re-established. It comprises of following events,
- Neutralization or enzymatic degradation of the various chemical mediators of inflammation;
- Normalization of vascular permeability;
- Cessation of leukocyte emigration, with subsequent death (by apoptosis) of extravasated neutrophils; and
- The necrotic debris, edema fluid, and inflammatory cells are cleared by phagocytes and lymphatic drainage.
Various mediators are released from leukocytes which facilitate the healing process. The primary role during healing is played by growth factors. Angiogenesis (formation of new blood vessels) takes place in the healing area that provides nutrients to various healing tissues. The proliferating fibro-blasts lay down collagen to fill defects, and residual tissue cells proliferate to restore structural integrity.
Chronic inflammation:
Acute inflammation may progress towards chronic inflamma-tion if the irritant is not removed. The chronic inflammation is characterized by the formation of granulation tissue which is an attempt to repair (discussed later).
Scaring:
Scarring is a type of repair after substantial tissue destruction. A scar is an area of fibrous tissue that replaces normal skin after an injury. These are several different types of scars, including keloid scars, which are the result of an overly aggressive healing process, contracture scars, which cause tightening of the skin, hypertrophic scars, which are raised and red scars that are similar to keloids but do not go beyond the boundary of the injury.
Systemic effects of acute inflammation
Acute inflammation may be accompanied by many systemic effects including,
Fever:
The most common effect of acute inflammation is pyrexia (fever). Fever is produced in response to substances called pyrogens that act by stimulating prostaglandin synthesis in the vascular and perivascular cells of the hypothalamus. Bacterial products, such as lipopolysaccharides (called exogenous pyrogens), stimulate leukocytes to release ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Acute-phase reaction:
Acute-phase proteins are plasma proteins, mostly synthesized in the liver, whose plasma concentrations may increase several hundred-fold as part of the response to inflammatory stimuli (see know more box on page 341).
Leucocytosis:
In acute inflammatory reaction, the leukocyte count may reach extraordinarily high levels of 40,000 to 100,000 cells/µl. These extreme elevations are referred to as leukemoid reac-tions because they are similar to the white cell counts obtained in leukemia. Various biological mediators such as IL-1 and TNF-α are strong stimulators of the release of cells from the bone marrow. Prolonged infection also induces proliferation of precursors in the bone marrow, caused by increased production of colony stimulating factors (CSFs).
Increased erythrocyte sedimentation rate (ESR):
There is an increased erythrocyte sedimentation rate due to increased production of acute phase proteins and reactants. In the presence of acute phase reactants (fibrinogen) erythrocytes aggregate due to the loss of their negative charge resulting in increased sedimentation.
Other manifestations:
Other manifestations of acute inflammation include increased heart rate and blood pressure, decreased sweating due to the redirection of blood flow from cutaneous to deep vascular beds, shivering, chills (perception of being cold as the hypothalamus resets the body temperature), anorexia etc. Severe infections may cause disseminated intravascular coagulation (DIC), metabolic disturbances including acidosis, and hypotensive shock. This clinical triad is described as septic shock. These precipitate due to the presence of a large amount of bacterial products in blood or the extravascular tissues, resulting in the secretion of high amount of various cytokines.
Chronic inflammation
Chronic inflammation, like acute inflammation is a host response to an inciting stimulus; however, there are some distinct differences. As the name indicates, “chronic” means prolonged duration- weeks to months. Along with this, chronic inflammation usually is productive or proliferative. Cells in the chronic inflammatory process tend to produce substances that add new tissue, such as collagen and new blood vessels. Many of these changes also represent the repair process and there is a blurry continuum between chronic inflammation and the whole repair process. It should be noted here that as compared to acute inflammation, rubor (redness) or calor (heat) are much less in chronic inflammation. Along with this, exudation is not a prominent feature as seen in acute inflammation. Because of the fibroplasia and neovascularization, areas affected by chronic inflammation tend to be slightly swollen and firm. The hallmark of chronic inflammation is ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Granulomatous inflammation
It is a distinctive pattern of chronic inflammation characteriz-ed by aggregates of activated macrophages with scattered lymphocytes. A granuloma is a focus of chronic inflammation consisting of a microscopic aggregation of macrophages that are transformed into epithelial-like cells, surrounded by a collar of lymphocytes and occasionally plasma cells. Granu-loma formation is usually regarded as a means of defending the host from persistent irritants of either exogenous or endogenous origin. The formation of a granuloma effectively “walls off” the offending agent and is, therefore, a useful defense mechanism. Granulomatous inflammation is observ-ed in the case of infections, allergic conditions, autoimmune conditions, neoplasm and conditions of unknown etiology. The granuloma is an area of active immunological activities consisting of numerous enzymes and cytokines such as platelet-derived growth factor, transforming growth factor-β, insulin-like growth factor, and TNF-α. As already stated, the predominant cells in granulomatous inflammation are ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Fracture healing
As discussed earlier in this chapter, following a soft tissue injury there is generation of an inflammatory reaction that is aimed at the healing of the injured area. Similar to the soft tissue injury, the hard tissue (bone) also responds to the injury by an inflammatory reaction, however, the events of healing differ. Bone can be injured from a variety of mechanisms, including trauma, infection, tumors, and compromised blood supply. Bone fracture healing is a very remarkable process because, unlike soft tissue healing, which leads to scar formation, the end result of normal healing is the regeneration of the anatomy of the bone and complete return to function.
A fracture describes a loss of continuity in the substance of a bone. Fractures in normal bones are usually caused by stress that exceeds the normal limits of tensile or compressive strain. The site of the fracture and the nature and direction of the fracture line in a bone depends on the magnitude and the direction of force applied on the bone. Fractures have been classified into the following types,
- Complete (both cortices broken, as seen on radiograph) or incomplete (one cortex broken).
- Displaced or undisplaced.
- Simple or comminuted (multiple fragments).
- Open (fracture communicates with skin surface) or closed.
Healing of bone fracture:
To facilitate appropriate healing, the fractured segments are approximated and stabilized. The fracture healing may be classified as primary fracture healing or secondary fracture healing. Primary fracture healing occurs when there is absolute stability (no motion between fracture surfaces under functional load), as found with anatomical reduction and rigid internal fixation. Secondary fracture healing occurs when there is relative stability (some controlled motion between fracture surfaces under functional load). The secondary frac-ture healing occurs in the following steps,
Hematoma formation:
Immediately following injury, there is hematoma (localized blood collection) formation within the fracture site. The blood collection is derived from the medullary cavity and the periosteum as well as tearing of soft tissue and adjacent muscle. As a result of the fracture, there is disruption of the Haversian systems with the death of osteocytes at the fracture surface. The degree of cell death at the site of injury depends on the degree of fracture comminution and displacement, amount of periosteal stripping and the extent of injury to the medullary contents.
Inflammation:
Similar to the events during soft tissue healing, an inflammatory reaction is triggered at the fracture site in which cells are attracted to the site of injury to mediate the inflammatory process and contribute to the replacement of necrotic bone with new bone matrix. The platelets and leukocytes and macrophages secrete inflammatory cytokines, such as interleukin 1 (IL-1) and IL-6, tumor necrosis factor alpha (TNF-α), and PGE2. These cytokines serve to attract progenitor cells that can differentiate into osteoblasts, endothelial cells, and osteoclasts. The activate cell migration, proliferation and differentiation of osteoprogenitor cells lead to repair. The inflammatory phase lasts 2-4 weeks after a fracture, and it overlaps with the next stage.
Repair:
The reparative phase occurs within the first few days, before the inflammatory phase subsides, and lasts for several weeks. The repair phase is characterized by the formation of a reparative “Callus”. The role of the callus is to enhance the mechanical stability of the site by supporting it laterally. Because initially there is an increased strain at the center of the fracture gap, new blood vessels cannot form, resulting in areas of low oxygen tension. As bone cannot form in areas of high strain or low oxygen tension; therefore, the formation of cartilage, which has low oxygen requirements, is favored. The local ischemia favors the differentiation of mesenchymal ……………….Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……………………..Content available in book……..
Once the soft callus has been deposited, hypertrophy of the chondrocytes takes place and callus remodeling begins. Under the influence of TNF-α, receptor activated nucleated kinase ligand, macrophage colony-stimulating factor, and matrix mineralizing proteins, there is activation of osteoclasts, macrophages, and chondroclasts which resorb the callus. Along with the resorption of cartilaginous callus, there is the creation of a woven bone callus that initiates at the peripheral regions and ultimately replaces the entire callus. The resultant ossified structure is known as “hard callus”. External callus forms on the outside of the fractured bone to bridge the gap. Internal callus forms more slowly from the medullary canal. Finally, the cortical continuity is restored.
Remodeling:
Callus is a rigid structure providing biomechanical stability, however, it does not fully restore the biomechanical properties of normal bone. The purpose of remodeling is to transform the hard callus into a lamellar bone structure with a central medullary cavity. It is the final phase of bone healing that goes on for several months. In remodeling, bone continues to form and becomes compact, returning to its original shape. There is an improvement in the blood circulation in the area. The result of the remodeling phase is a gradual modification of the fracture region under the influence of mechanical loads until optimal stability is achieved. This phase is biochemically orchestrated by IL-1 and TNF-α, which show high expression levels during this stage, as opposed to most members of the TGF-β family which have diminished in expression by this time. Along with this, bone morphogenetic proteins, such as BMP-2 are also highly expressed during this phase of healing.
Conclusion
The inflammation is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molecular mediators. Its function is to eliminate the initial cause of cell injury, clear out necrotic cells and tissues damaged from the original insult and the inflammatory process, and initiate tissue repair. As dental professionals, we are exposed to this phenomenon on a daily basis as most of the patients report to the dentist with pain and swelling. Thus, an in depth knowledge of inflammatory response is an essential component of our learning process.
References
References are available in hardcopy of the website “Textbook of basic sciences for MDS students”.
Periobasics: A Textbook of Periodontics and Implantology
The book is usually delivered within one week anywhere in India and within three weeks anywhere throughout the world.
India Users:
International Users:

